Multi-layer Regulation of Rubisco in Response to Altered Carbon Status in Synechococcus elongatus PCC 7942
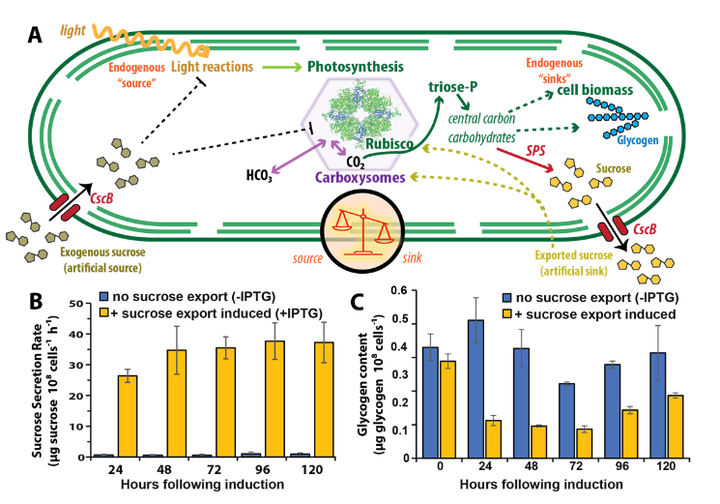 Rubisco activity and organization are key outputs involve in source-sink balancing in cyanobacteria
Rubisco activity and organization are key outputs involve in source-sink balancing in cyanobacteria
Abstract
Photosynthetic organisms possess a variety of mechanisms to achieve balance between absorbed light (source) and the capacity to metabolically utilize or dissipate this energy (sink). While regulatory processes that detect changes in metabolic status/balance are relatively well-studied in plants, analogous pathways remain poorly characterized in photosynthetic microbes. Herein, we explore systemic changes that result from alterations in carbon availability in the model cyanobacterium Synechococcus elongatus PCC 7942 by taking advantage of an engineered strain where influx/efflux of a central carbon metabolite, sucrose, can be regulated experimentally. We observe that induction of a high-flux sucrose export pathway leads to depletion of internal carbon storage pools (glycogen), and concurrent increases in photosynthetic parameters. Further, a proteome-wide analysis and fluorescence reporter-based analysis revealed that upregulated factors following the activation of the metabolic sink are strongly concentrated on ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) and axillary modules involved in Rubisco maturation. Carboxysome number and Rubisco activity also increase following engagement of sucrose secretion. Conversely, reversing the flux of sucrose by feeding exogenous sucrose heterologously results in increased glycogen pools, decreased Rubisco abundance, decreased photosystem II quantum efficiency, and carboxysome reorganization. Our data suggest that Rubisco activity and organization are key outputs connected to regulatory pathways involved in metabolic balancing in cyanobacteria.